Description
TECHNICAL SPECIFICATIONS
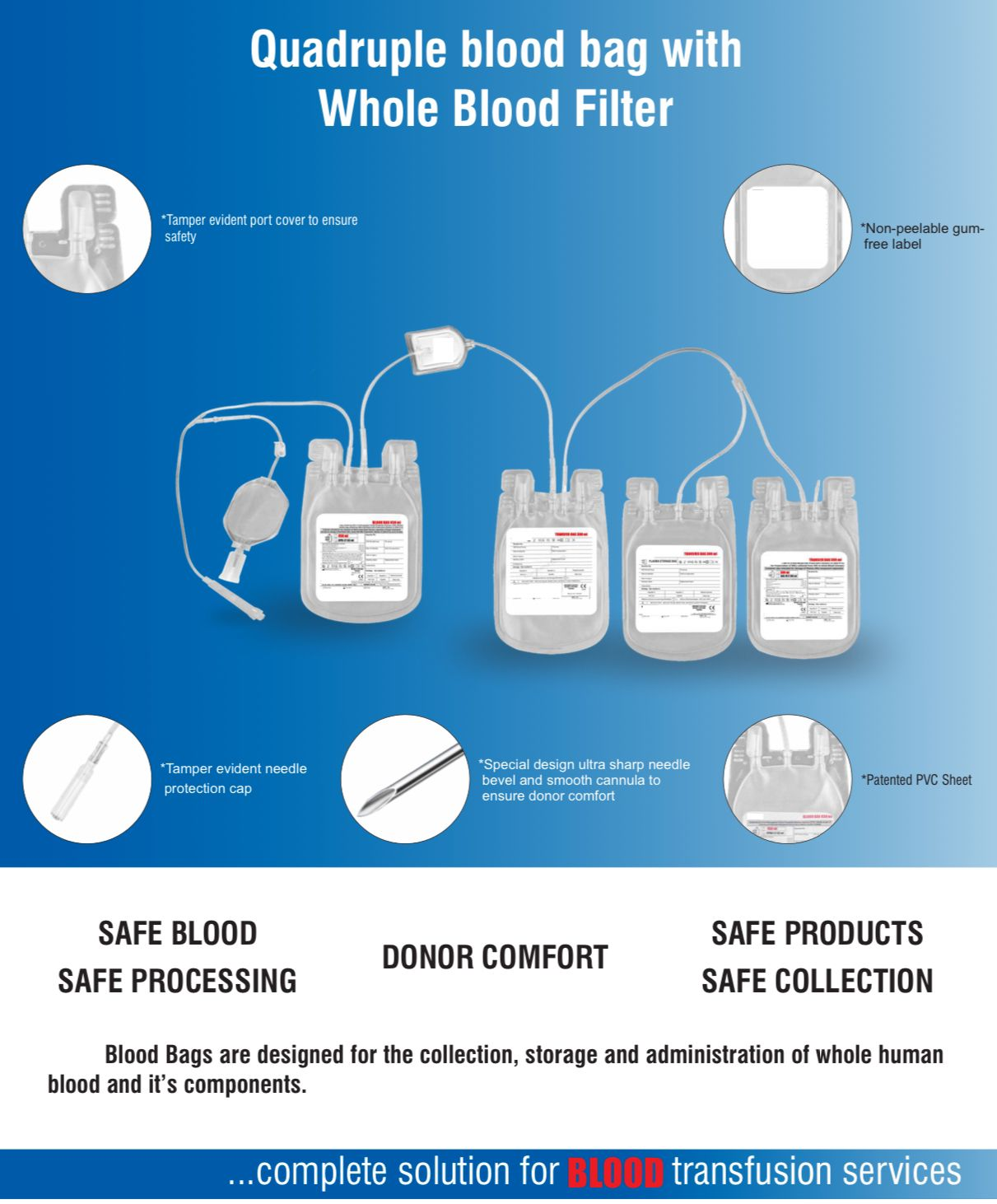
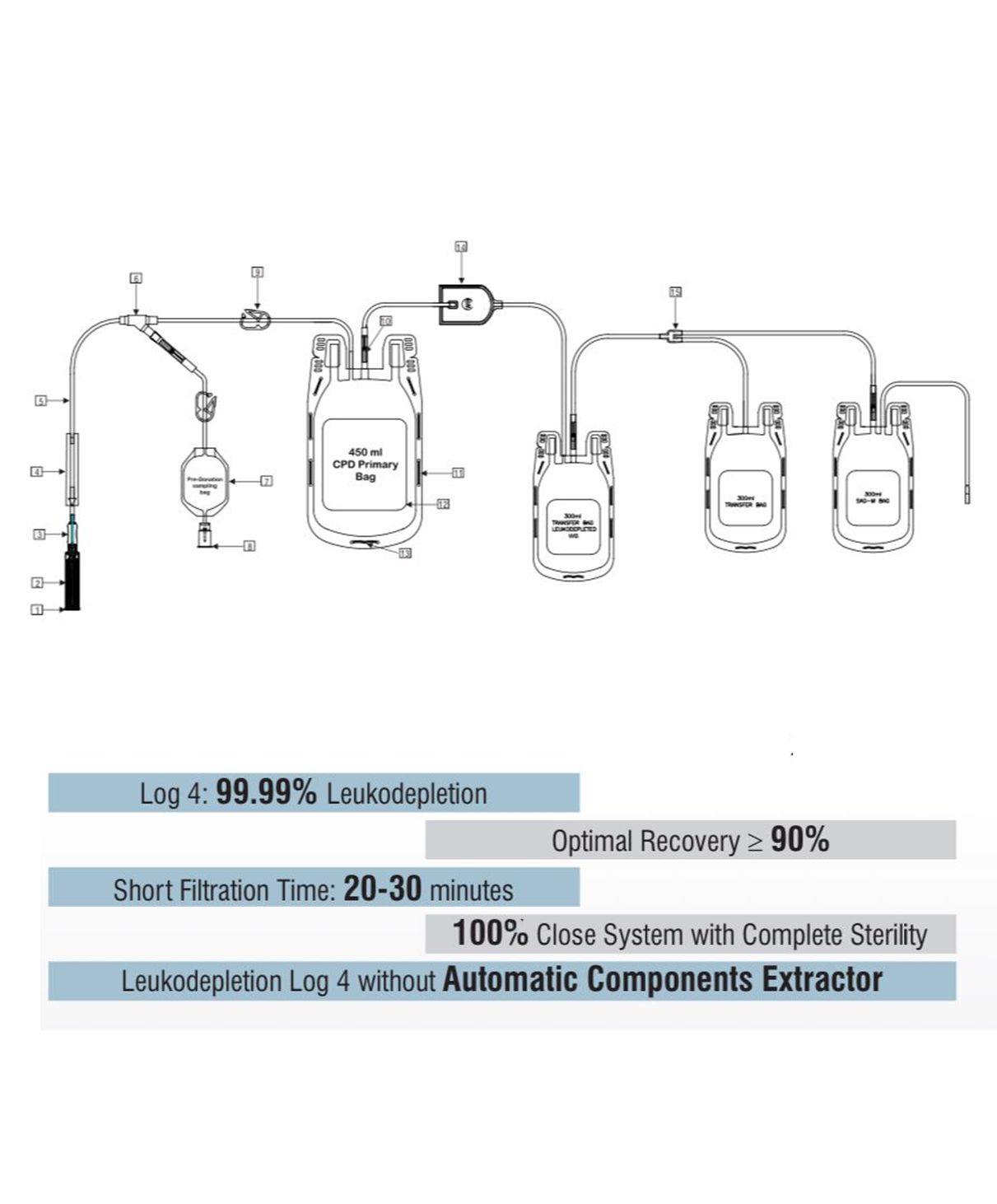
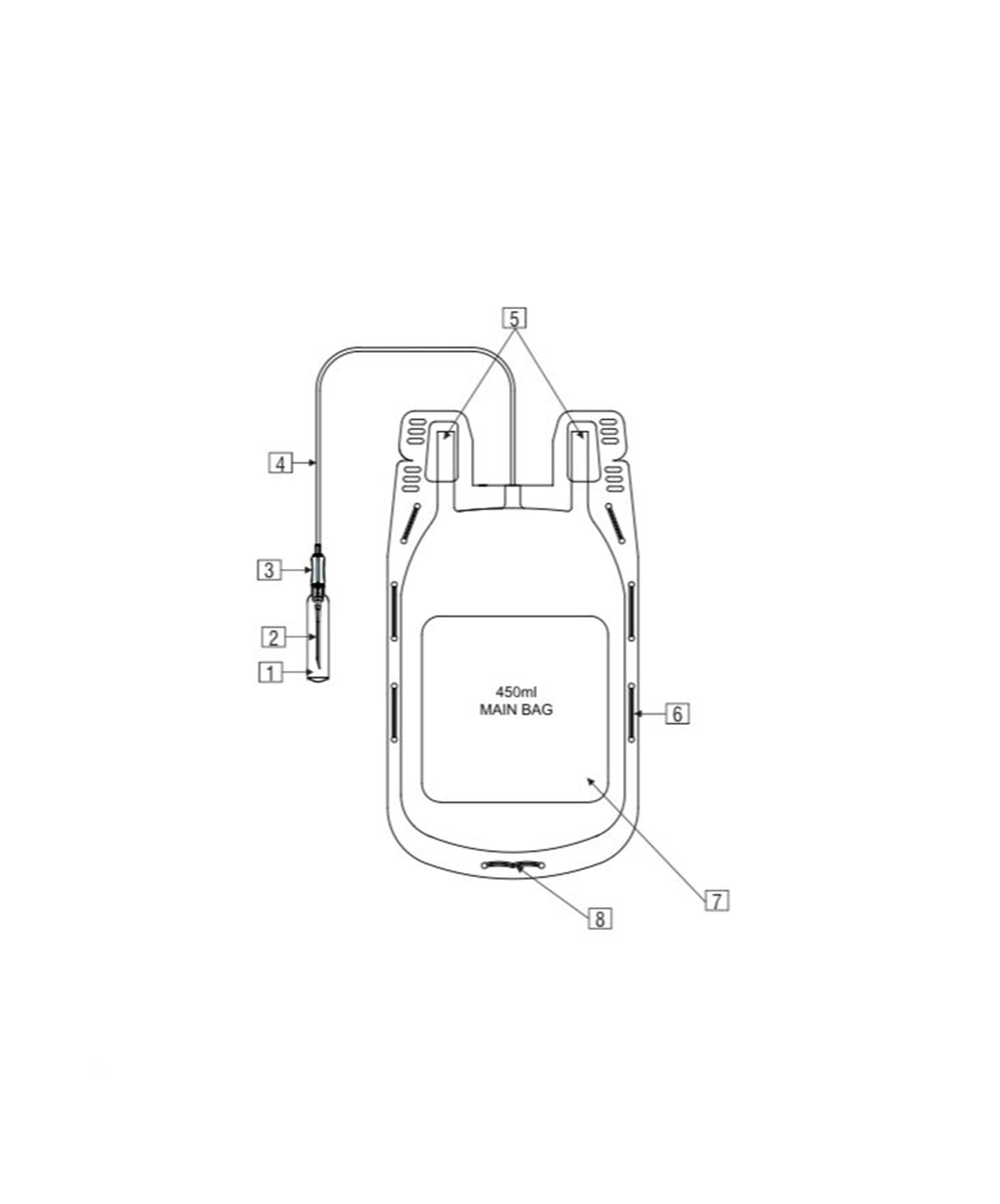
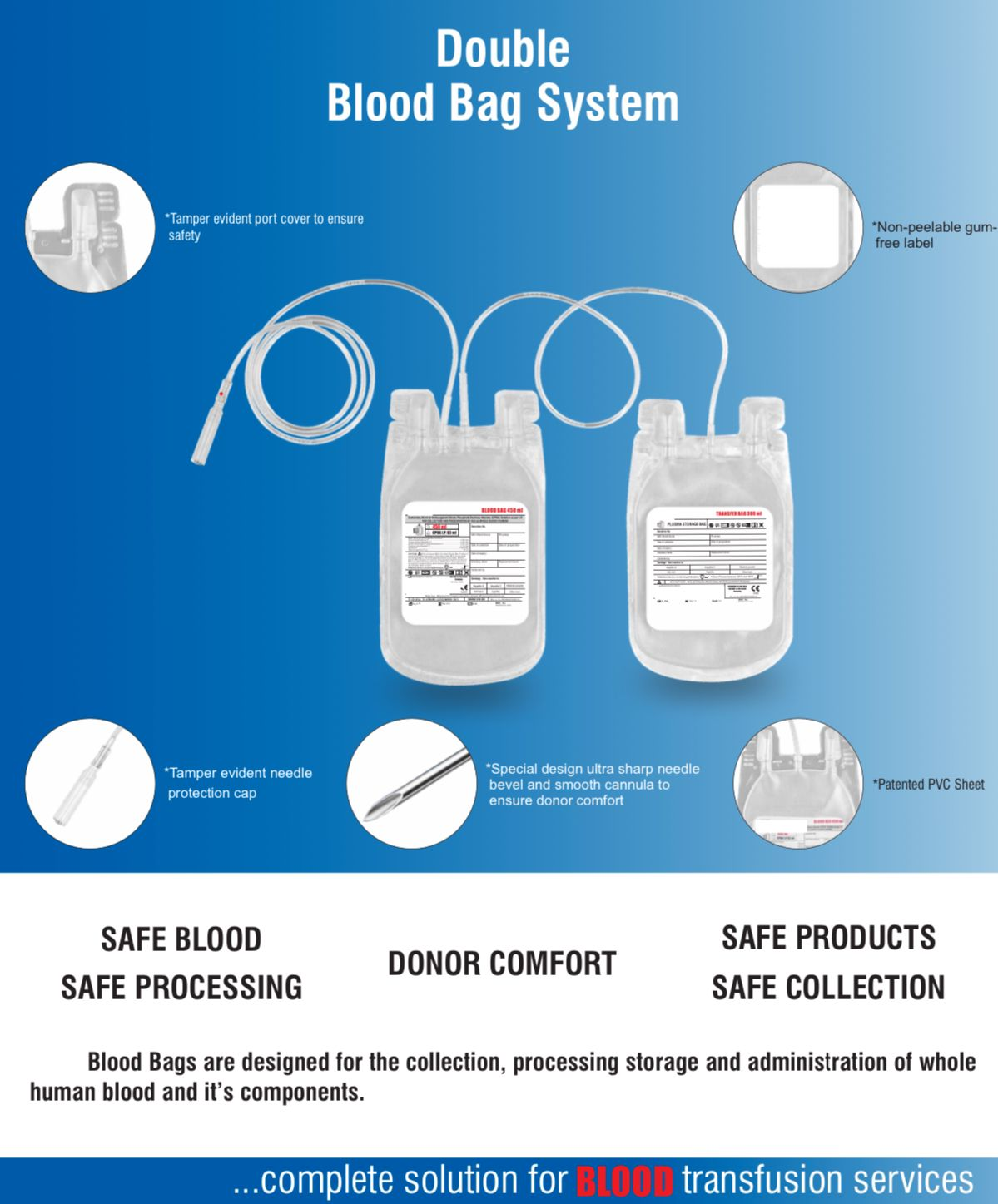
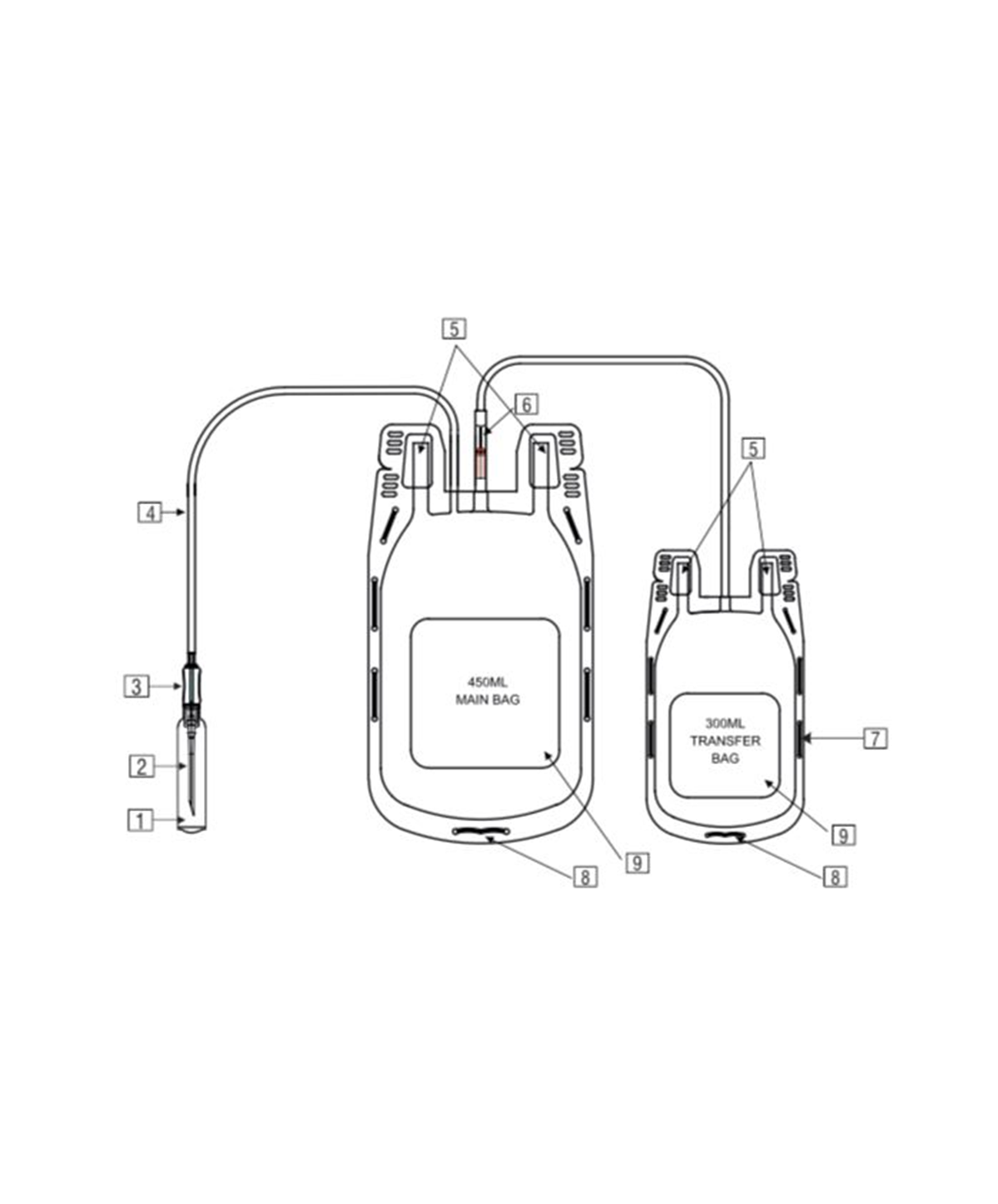
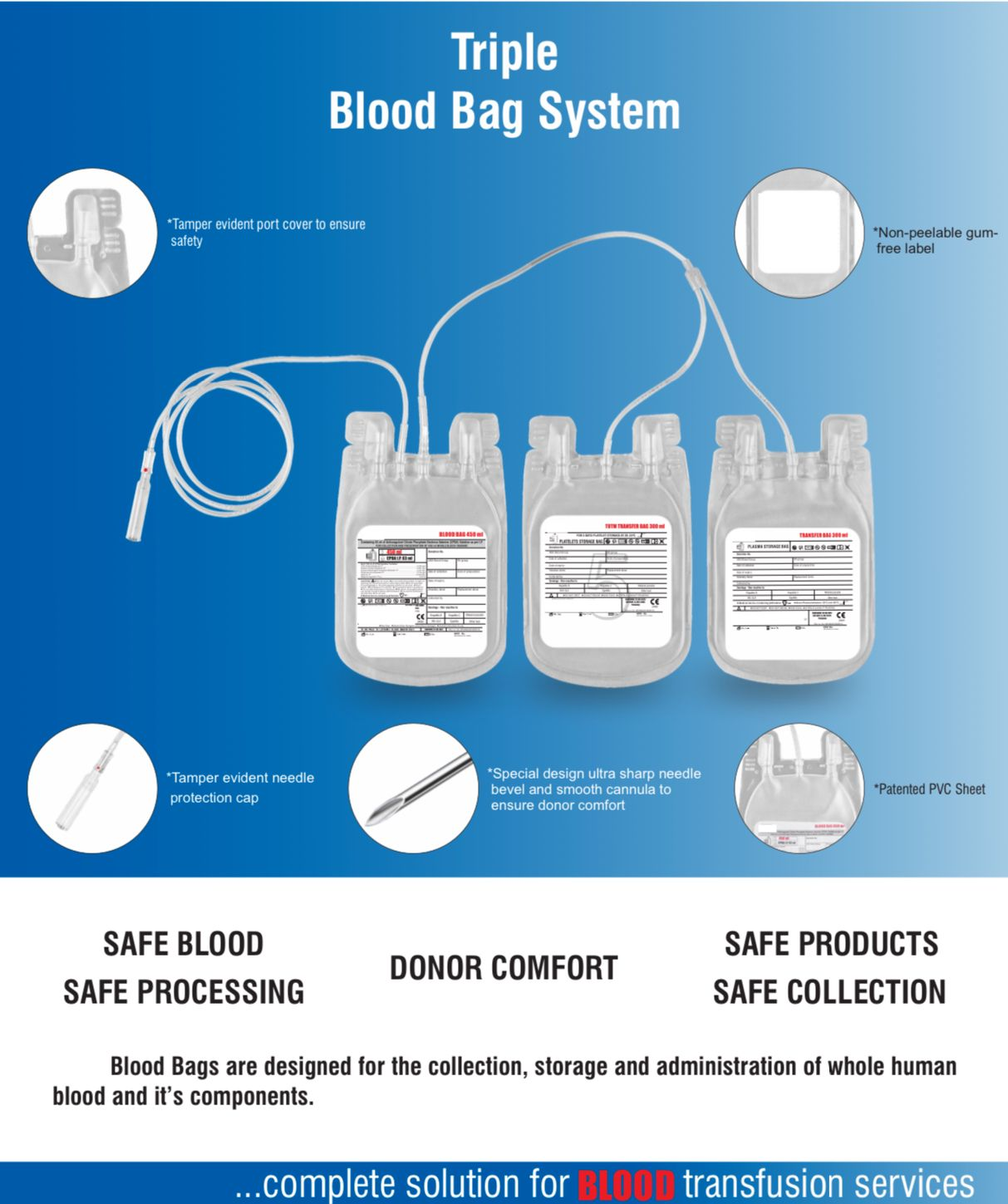
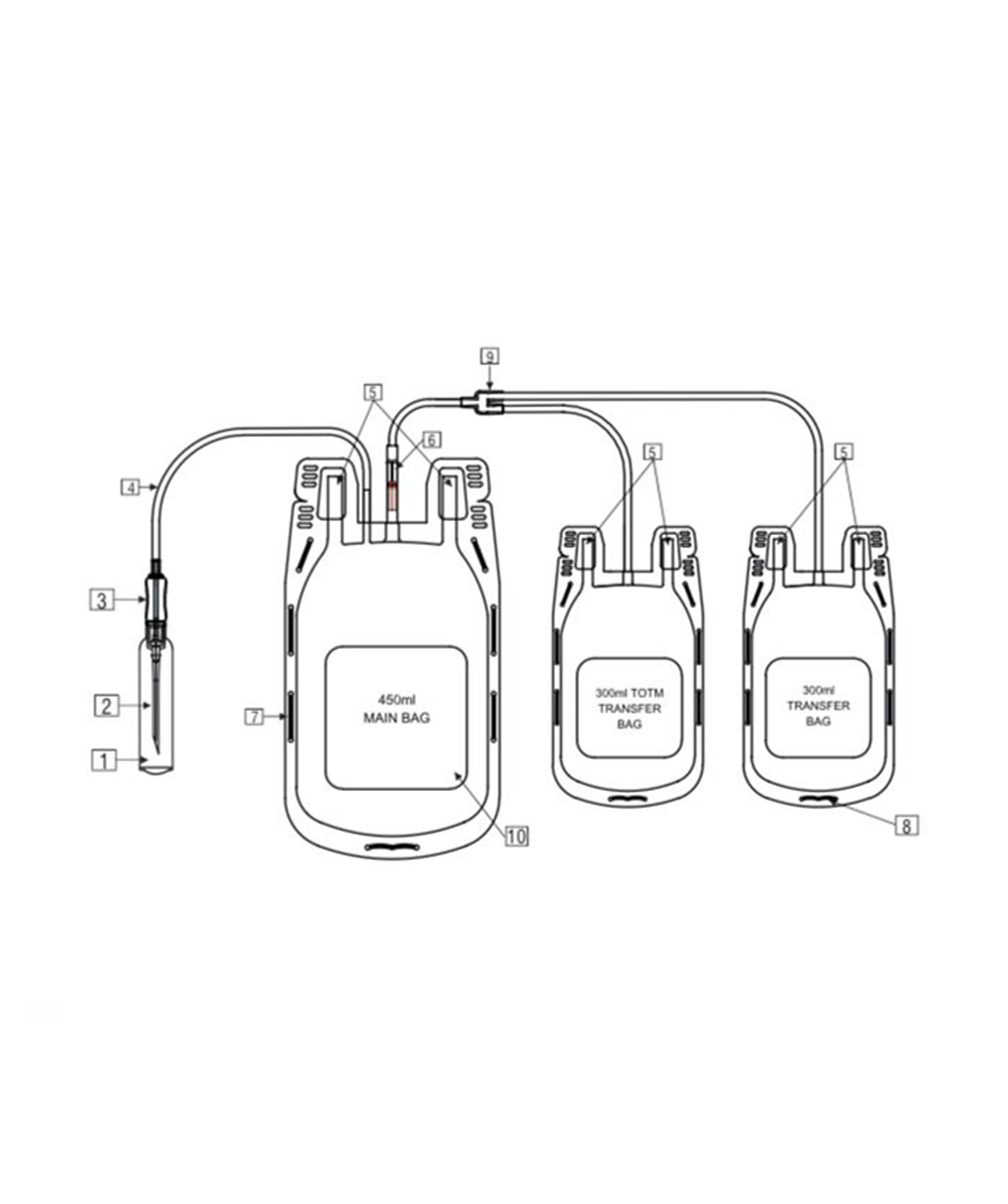
- Quadruple blood bag with Whole Blood filter 350 / 450 / 500 ml, containing 49 / 63 / 70 ml of CPD anticoagulant solution and SAG-M as preservative solution 78 / 100 / 111 ml.
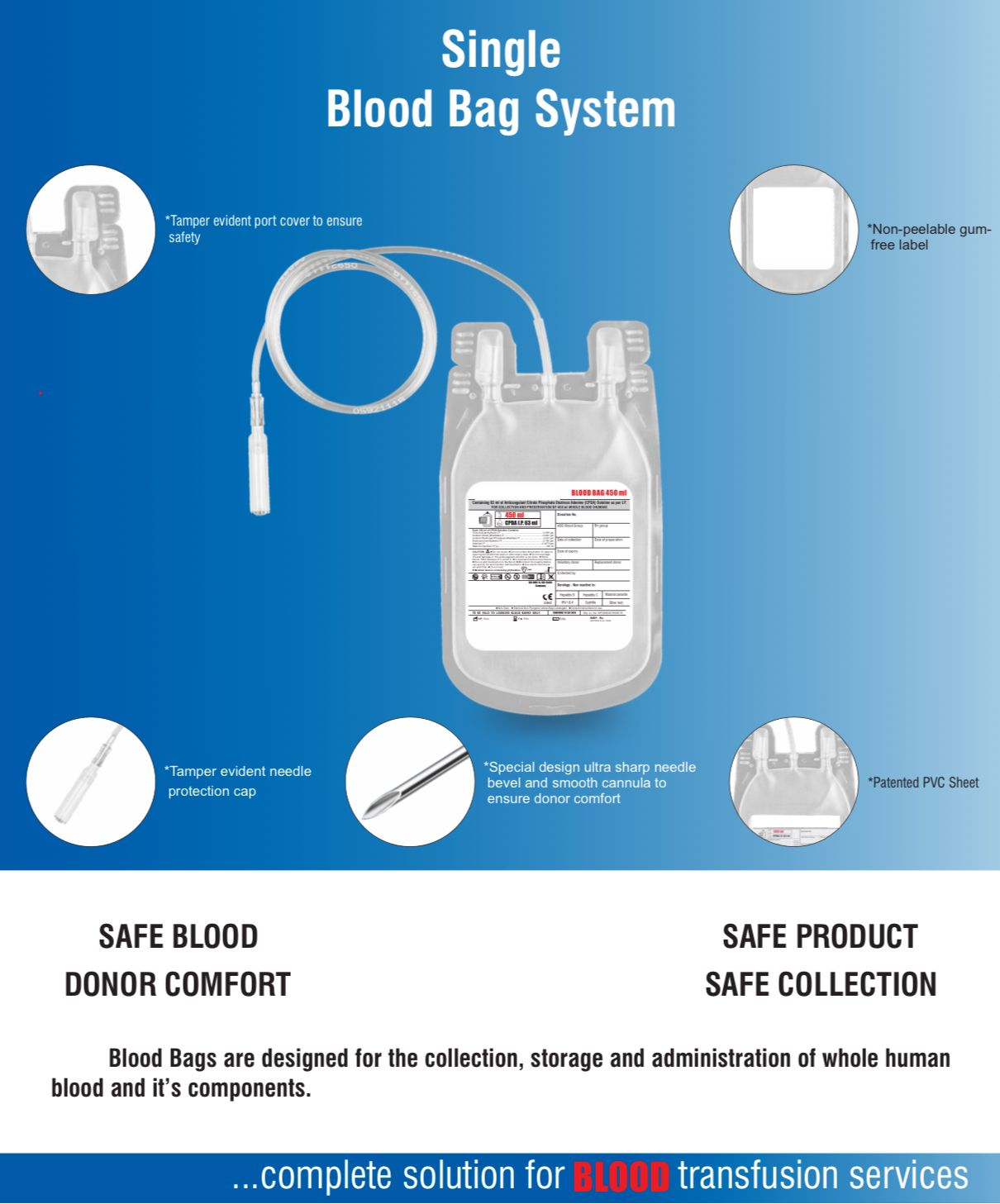
- DEHP plasticized PVC, collapsible, non-vented, sterile container complete with collecting tube in a closed system.
- Sterile non-pyrogenic, non-toxic, non-hemolytic, biocompatible material.
- The inner bag maintains sterility.
- No risk of contamination & air embolism with all leakproof.
- Slits on both sides to accommodate 5-10 ml volume test tube & at the bottom of the bag to hang the blood bag during transfusion.
- Flexible, non-sticking, transparent, leak-proof tubing having multiple printed & ID/Segment numbers.
- 16 G ultra thin-walled, needle triple bevel design, sharp, rust-proof, tightly fixed with hub & tamper proof needle cover.
- Tamper proof & easily accessible external port.
- Hot melt technology non-peelable, heat-sealed label with transparent adhesive remarked attached between room temperature to -800
- 3 years shelf life from the date of manufacturing.
- Maintain biochemical parameters of leukodepleted packed Red Blood Cells(PRBC) for up to 42 days in SAG-M and plasma(FFP) for up to 1 year.
- Protective dual packaging(CPP & Aluminum)
- Blood bags meet all the requirements as laid down in ISO 3826.
- Individual plastic blood bags packed in plastic CPP pouches & such 2 plastic pouches packed in an aluminum foil pack. 10 such aluminum foil packs were further packed in corrugated boxes.