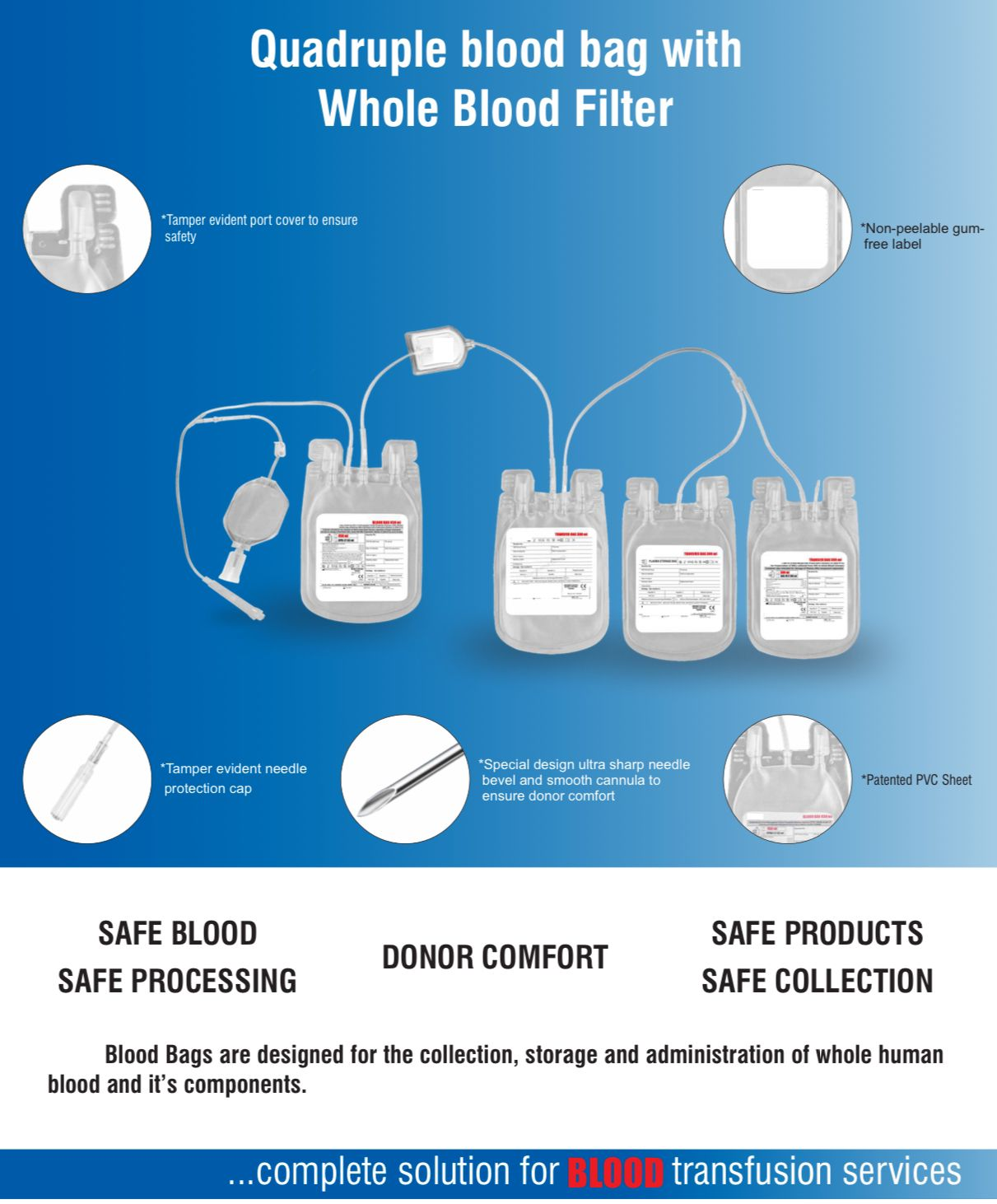
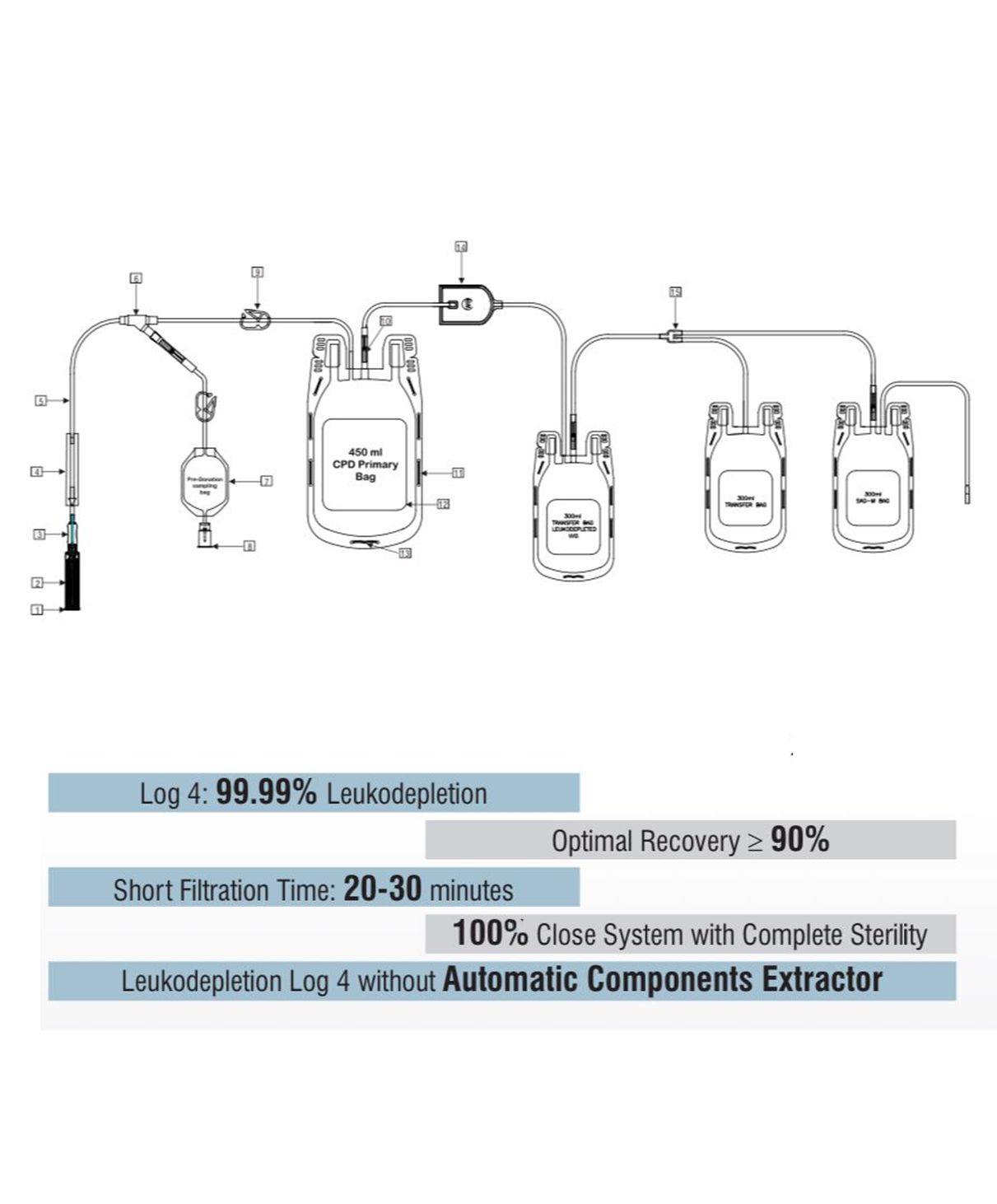
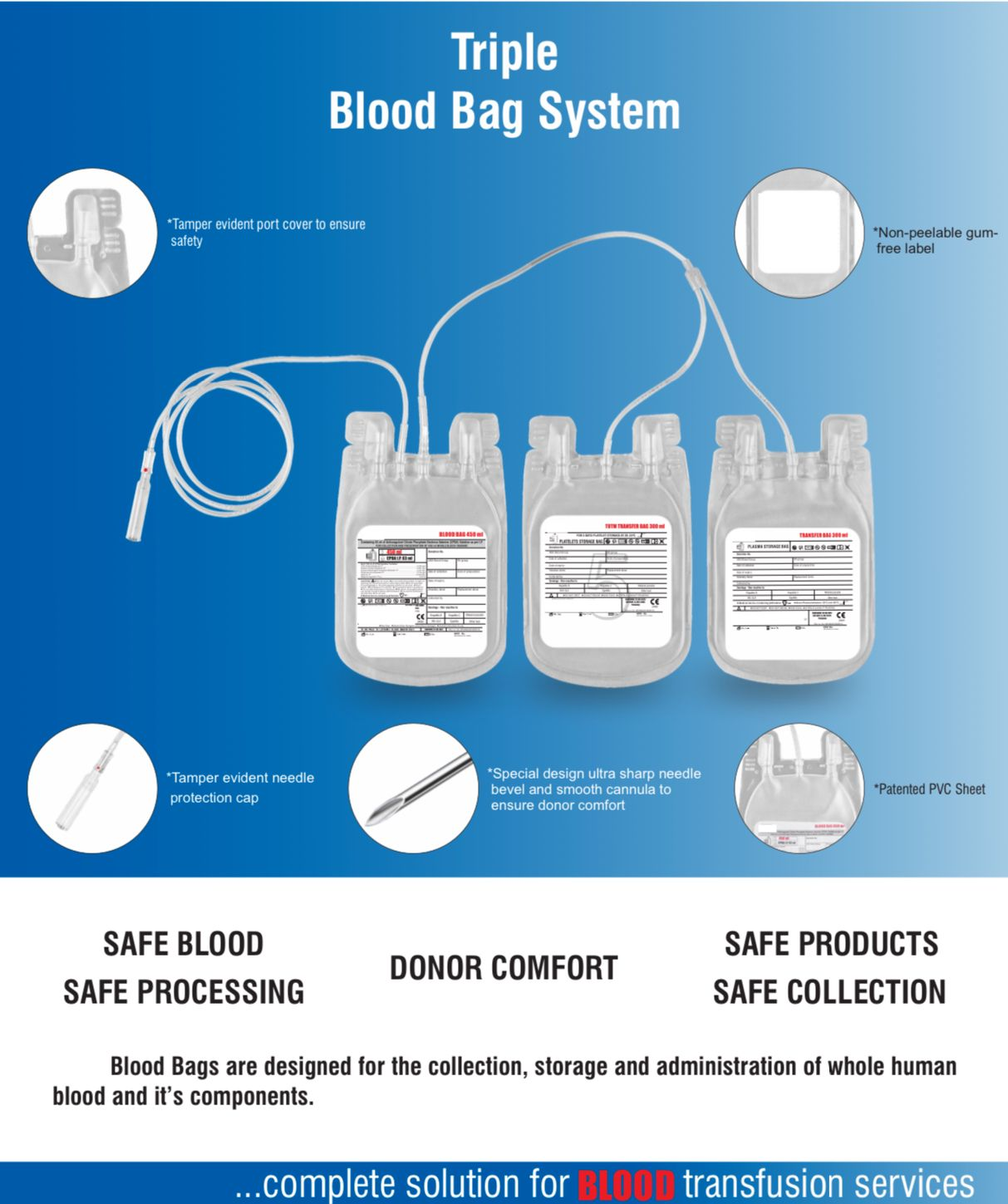
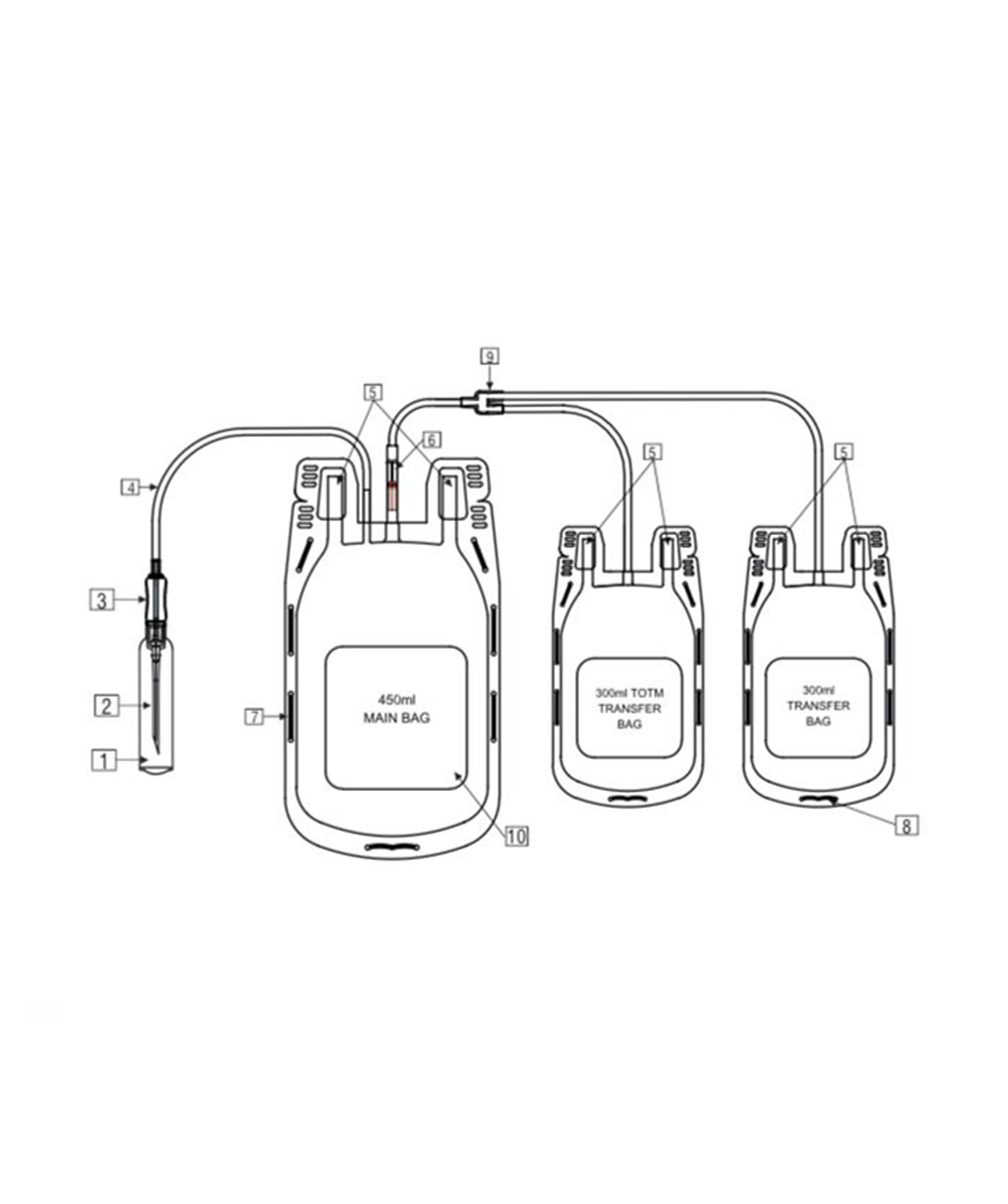
Description
TECHNICAL SPECIFICATIONS
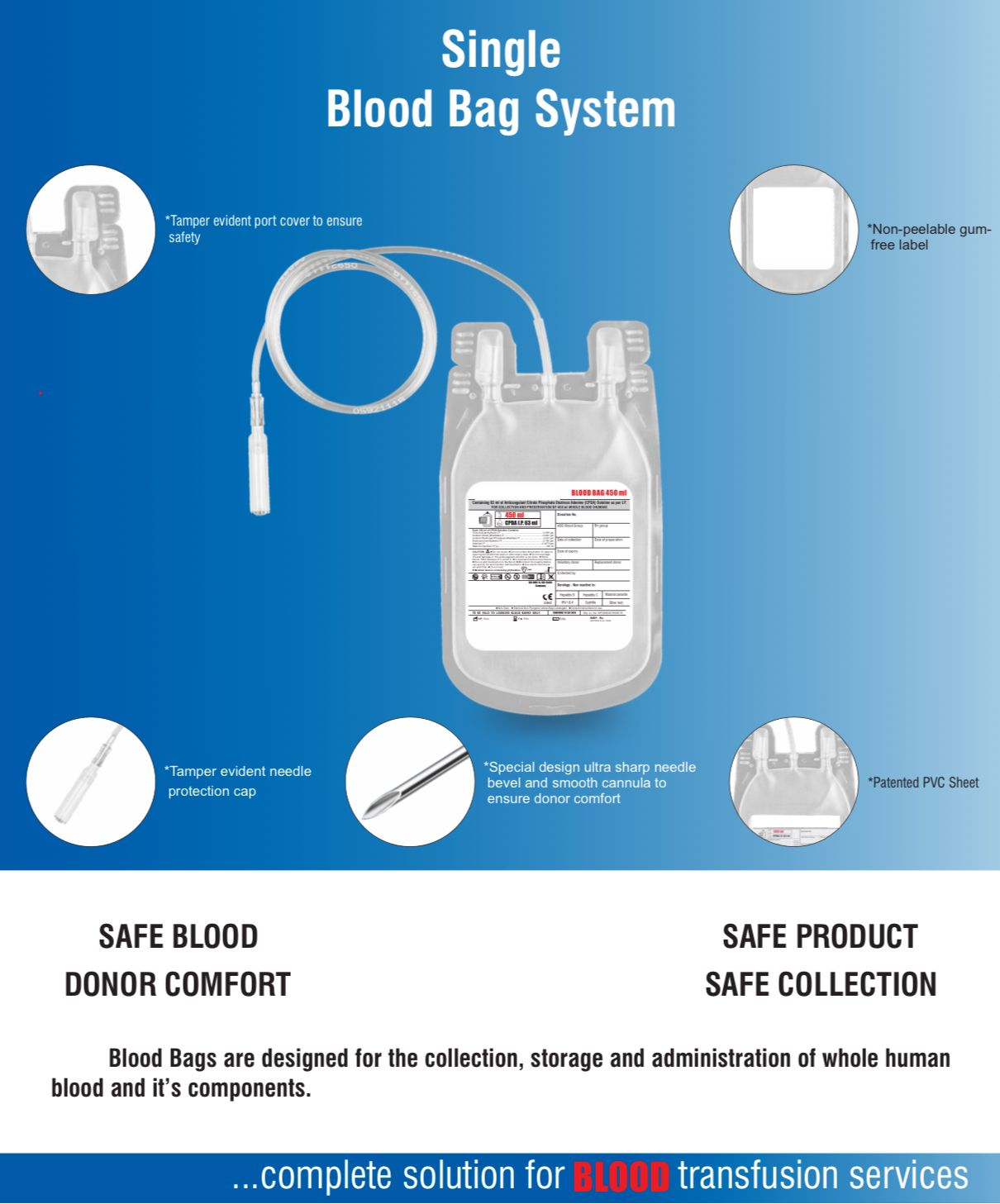
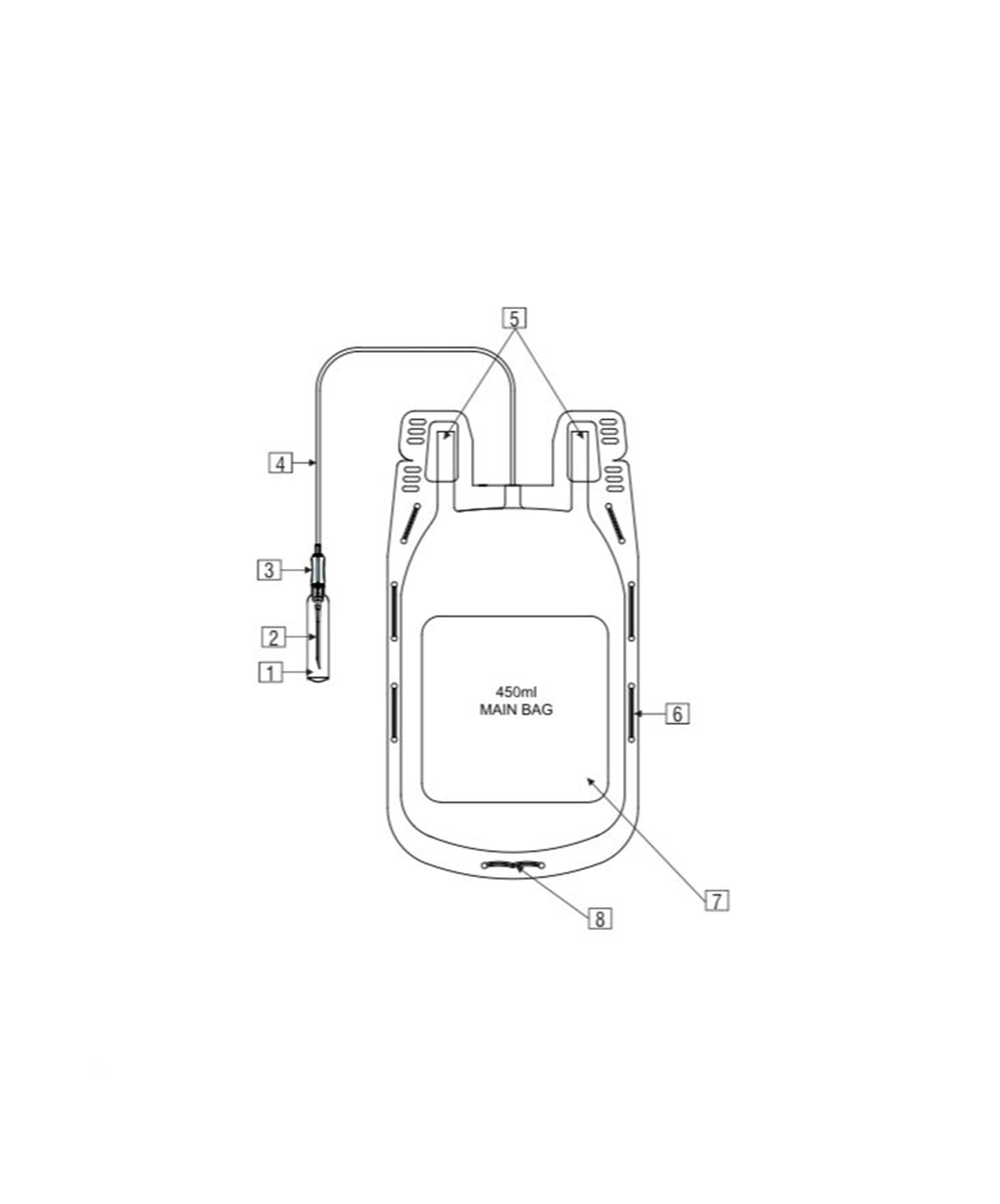
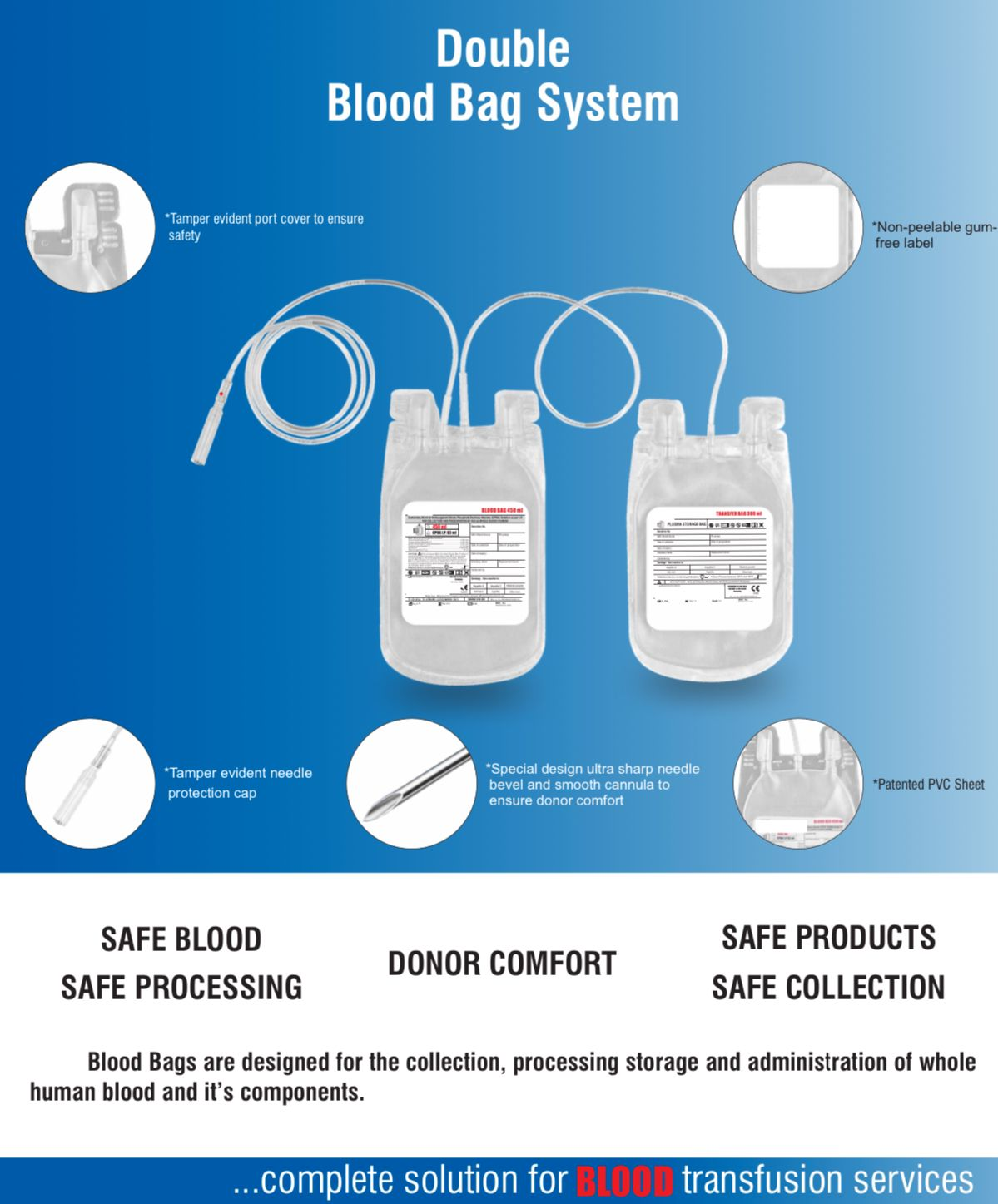
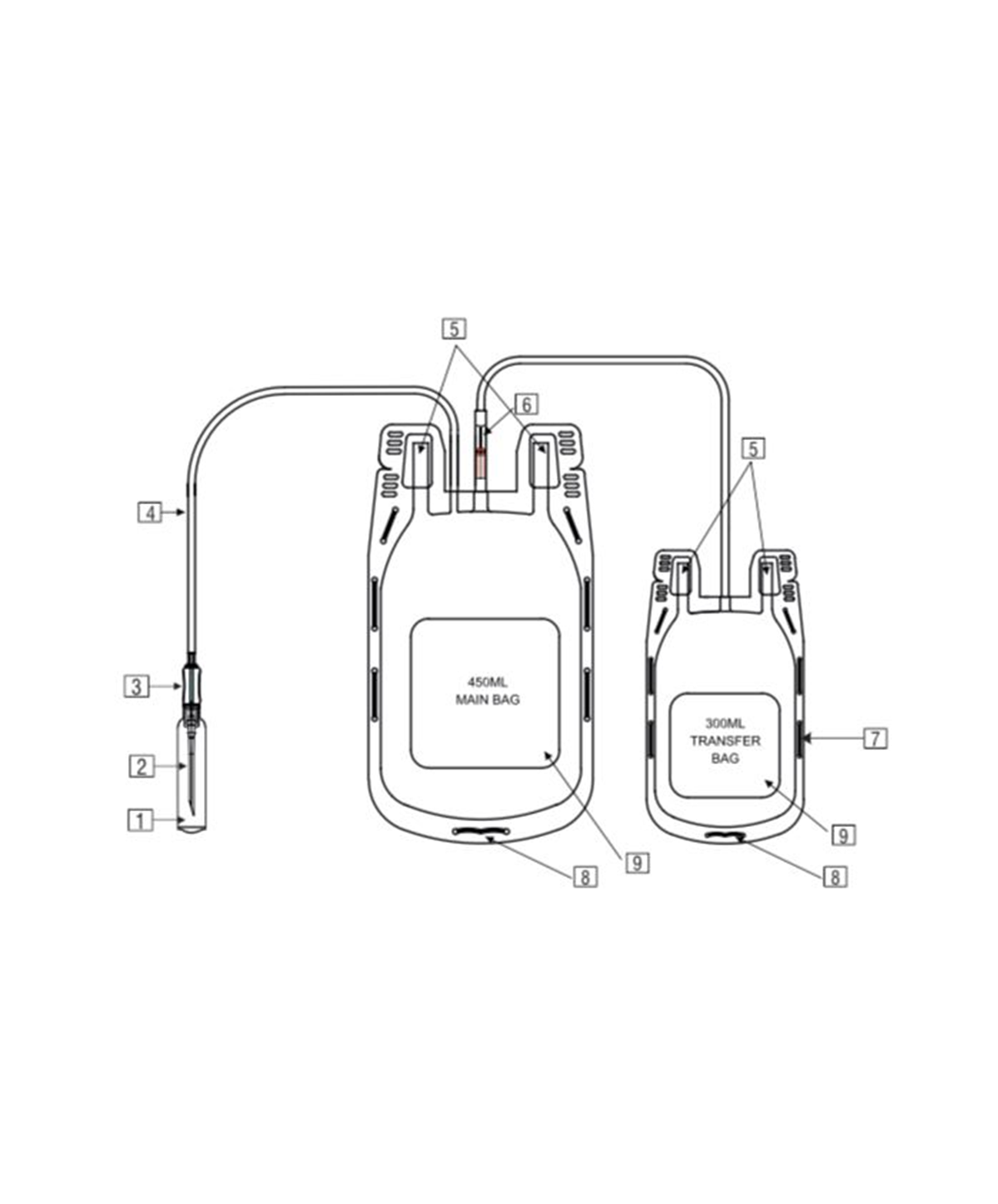
- Single Blood Bag – 100 / 250 / 350 / 400 / 450 / 500 ml, containing of 14 / 35 / 49 / 56 / 63 / 70 ml of CPDA/CPDA-1 anticoagulant solution.
- DEHP plasticized PVC, collapsible, non-vented, sterile container complete with collection tube in a closed container.
- Sterile non-pyrogenic, non-toxic, non-hemolytic, bio-compatible material.
- No risk of contamination & air embolism. Leak-proof technology.
- Slits on both sides to accommodate 5-10 ml volume test tube & at the bottom of the bag to hang the blood bag during transfusion.
- Flexible, non-sticking, transparent, leak-proof tubing having multiple printed & id /segment numbers.
- 16 G ultra thin-walled, needle, triple bevel design, sharp, rust-proof, tightly fixed with hub & tamper proof needle cover.
- Tamper proof & easily accessible external ports.
- Hot melt technology non-peelable, heat-sealed label with transparent adhesive remarked attached between room temperature to -800
- 3 years shelf life from the date of manufacturing.
- Maintains biochemical parameters of whole blood for up to 35 days of storage.
- Protective dual packaging (CPP & Aluminum)
- Blood bags meet all the requirements as laid down in ISO 3826.
- Individual PVC (Plastic Bags) packed in plastic CPP pouches & such as 10 plastic pouches packed in an aluminum foil pack. 10 such aluminum foil packs were further packed in corrugated boxes.
SPECIAL FEATURES ON DEMAND
Blood Bags are available with various combinations of safety features including a needle protection device and a pre-donation sampling pouch with a luer adapter.